Out of the 70% of water, which is what makes 2/3 of the earth, only 3% is fresh and indeed 0.5% of itself remains available for us while the remaining 2.5% gets locked in glaciers, icebergs. While still there exists people wasting drinking-water, 2.2 billion people are left with no clean-water to drink around the world.
Though 70% of world is water, it’s not drinkable as it’s salty-water and the desalination of salty water costs expensive, preventing it from being widespread in the world. MIT Researchers, however, came out with a device that sizes to a suit-case and easily converts Sea Water to Drinking water consuming less energy than that of conventional water-filters.
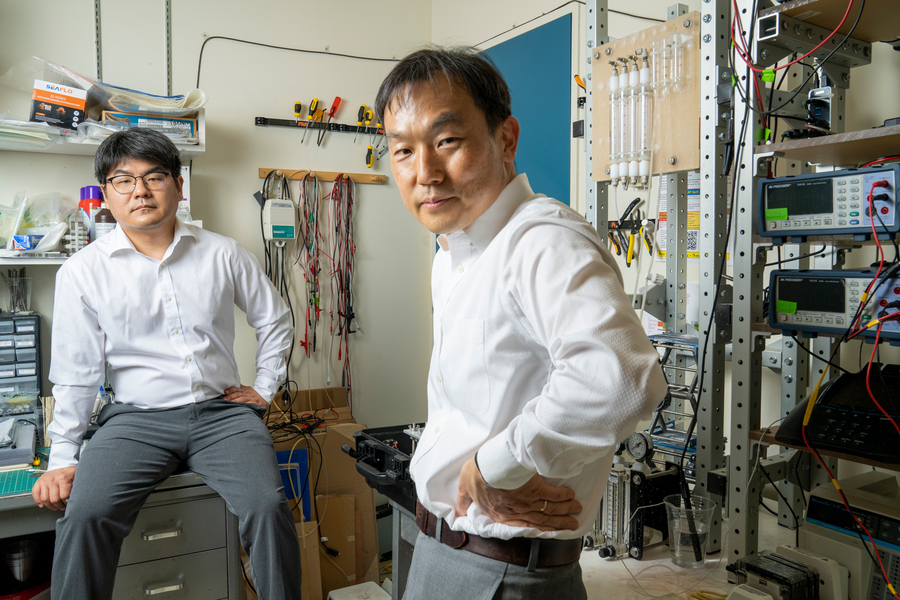
Jongyoon Han, a professor of Electrical engineering and computer science and of biological engineering, along with his team, have developed a portable desalination unit in the size of a suit-case that is capable of removing unwanted particles and salts to fetch WHO standard’s drinking water. The device weighs less than 10 kilograms, making it easier to tote.
The device differentiates itself from nominal water-filtering devices by deploying a unique way of separation of salts and particles from water – through electrification of water. It automatically generates drinking water that even exceeds World Health Organization’s quality standards of water by applying electrical power to sea-water. It serves so, with just a push of a button. Han says that the device requires less power than your cell-phone charger to operate, which can also be run by solar-panel, portably affixed to its back.
How it Works?
Water-filters which are existing commercially now, incorporate high-pressure pumps to push water through filters, needing for bulky-space and power to run smoothly. This also constraints of being miniaturized, without sacrificing few percentages of its energy-efficiency.
On the contrary, this new breakthrough by MIT scientists called by ‘Ion Concentration polarization (ICP)’ method, applies electrical field to membranes place above and below a channel of water. The electrified membranes repel positively or negatively charged particles – including salt molecules, bacteria and viruses and any other unwanted particles – as they flow past. The charged particles are funneled into a second stream of water that is eventually discharged, pouring out freshwater remains to drink.
“This is really the culmination of a 10-year journey that I and my group have been on. We worked for years on the physics behind individual desalination processes, but pushing all those advances into a box, building a system, and demonstrating it in the ocean, that was a really meaningful and rewarding experience for me,” says senior author Jongyoon Han
Still, ICP does not vanishes all salts floating in the middle of the channel. So, electrodialysis was introduced as a second process to remove remaining salt ions.

Owing to increase its efficiency and scalability, researchers collaborated to furnish out finally with two-stage ICP process, with water flowing through six modules in the first stage then through three in the second stage, followed by a single electrodialysis process. This minimized energy usage while ensuring the process remains self-cleaning.
“While it is true that some charged particles could be captured on the ion exchange membrane, if they get trapped, we just reverse the polarity of the electric field and the charged particles can be easily removed,” Yoon explains.
The Seawater-to-drinking water Converting Device
Research had been completed and the time had arrived to test the device, for which the researches field-tested at Boston’s Carson Beach.
Junghyo Yoon (research scientist in RLE) and Sungku Kang (Postdoc at Northeastern University) set the suitcase near the shore and tossed the feed tube into the water. In about half an hour, the device tapped clear, drinkable water in a drinking cup.
Related Posts
“It was successful even in its first run, which was quite exciting and surprising. But I think the main reason we were successful is the accumulation of all these little advances that we made along the way,” Han says.
The prototype fetched drinking water at a rate of 0.3 litres per hour, and requires only 20watt-hours per litre.
“Right now, we are pushing our research to scale up that production rate,” Yoon says.
When fully scalable, the device could be a great resource in remote areas, such as communities on small islands or aboard seafaring cargo ships and to the possible, circumvent thirstiness for coastal cities.