Onset of electricity back from the nineteenth century, there have been consistent inventions, innovations, reengineering and revolutionary aspects in the energy and life. And once occurred, the discovery of ‘superconductivity’ in 1917 by a Dutch physicist Heike Kamerlingh Onnes, wherein he observed mercury to behave differently at 4.20 above absolute zero (-268.80 C). This awed the world, increasing the research and works at depths in the field. But the hardships of the field, kept the discovery bootless for almost a century (of-course with few advancements over the years).
The on-hold discovery would get a new pace of revolution, under a complete success. Energy transmission would be 100% efficient, maglev trains wouldn’t be harder and interestingly, your smartphones doesn’t need batteries anymore. But “when”?
Ranga Dias team of physicists of University of Rochester had made a new feat onto this field; can be claimed a revolutionary, as it may answer the “when”, in the near future! “This is a landmark”, says University of Cambridge’s Physicist, Chris Pickard.
Superconductivity
Let’s start here, to have a better grasp about the revolution.
Superconductivity is a phenomenon whereby a charge moves through a material without resistance. Normally circuits involve some sort of loss of energy as heat or other, due to resistivity of the material. In cases of superconductivity, the materials exhibit zero resistance to the flow of electrons, ultimately putting down the dissipation of energy via undesirable forms.
How? Generally, electrons divest their repulsive force to each other and said to snuggle or conjoin with one another (cooper pairs), if the atoms are subjected to cresty pressure and troughy temperature. In this cold low-energy state, electrons behave uncertainly losing their identity, which makes them to slip off through the crowd of atoms with ease. Thusly, an electron deforms its material structure, forging a new structure, while adhering with another electron, fitting as ‘superconductive electron’ and ‘superconductors’ as in whole.‘BCS theory’ construes the same.
What’s the difficulty?
The real hurdle of superconductivity to be real is its likely atmosphere, which requires a two-third pressure of the center of earth and unideal temperature (say, beneath 00 C). Materials seen to behave superconductively only under this condition. Researches are under diligence, trying out with different elements and compounds and circumstances for the aim of achieving superconductivity under room-temperature.
After few decades of discovery of superconductivity, in 1986 physicists were able to bring down the temperature to 30K while working out with copper oxide ceramics and the temperature called to be as critical temperature (Tc) of the material. Several researches and scientist manifolded different materials and other ceramics to perceive the most conforming. By 1994, they pushed the Tc up to 164 K in a mercury-based copper oxide under pressure.
Neil Ashcroft, a theorist at Cornell University had suggested a different material to exhibit superconductivity above room temperature: Metallic hydrogen, i.e., hydrogen under intense pressure. Hydrogen, a gas in ambient atmosphere, solidifies as metallic hydrogen when consigned to high pressure, a pressure surpassing that of at the center of earth. In such a spell, hydrogen under experiments between diamond anvil cells for crushing, possibly breaks the diamonds.
But, in 2004, the same theorist came with another tip of pre-compressing hydrogen could pave the way for superconducting hydrogen at higher temperature and relatively lower pressure. This worked.
In 2015, Mikhail Eremets at Max Planck Institute and his team colleagues resolved the issue by drawing upon Sulphur hydride (H3S), whereby they achieved superconductivity at 203K and 155 gigapascals (GPa). Adding lanthanum to hydrogen-rich compounds brought up the temperature to 250K. This was the last achieved feat of the field.
First room-temperature Superconductor
Presently, Ranga Dias discovered that a third material to hydrogen and sulphur, namely carbon, could potentially moot the process and it really happened. At first, treating the sample at 148GPa via electrical leads, they found it superconducting at 147K. And by increasing the pressure to 267GPa, resistivity surprisingly hit zero at an ideal winter temperature 287K (nearly 150 C). Henceforth, carbonaceous sulphur hydride examined superconductivity at room-temperature under extreme pressures.
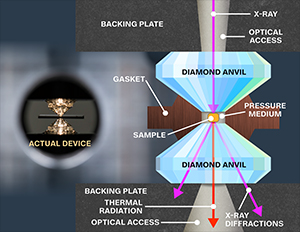
Dias and his colleagues are now working to produce their material at lower pressures. “Take diamond: it is a high-pressure form of carbon, but nowadays you can grow it in a lab with chemical deposition techniques,” says Dias. “It used to require high pressure, but now we can grow it – we may be able to do something similar with superconductors.”
What happens if this occurs?
Ideally, superconductors at room-temperature and normalizing pressures makes a ‘paradigm shift’ along this developing world. “We live in a semiconductor society, and with this kind of technology, you can take society into a superconducting society where you’ll never need things like batteries again,” says Ashkan Salamat of the University of Nevada Las Vegas, a coauthor of the discovery.
Superconductivity also possess magnetic field to pass through the material, making it possible to levitate such materials, an origin for levitating magnetic trains, called maglev trains.
The best applications are recapped here:
- Power grids that transmit electricity without the loss of up to 200 million megawatt hours (MWh) of the energy that now occurs due to resistance in the wires
- A new way to propel levitated trains and other forms of transportation, including hyperloop.
- Medical imaging and scanning techniques such as MRI and magnetocardiography
- Faster, more efficient electronics for digital logic and memory device technology
& The Best is Yet to Come!
References:
- https://www.newscientist.com/article/2256953-first-room-temperature-superconductor-could-spark-energy-revolution/?utm_campaign=later-linkinbio-newscientist&utm_content=later-11009729&utm_medium=social&utm_source=instagram
- https://www.sciencemag.org/news/2020/10/after-decades-room-temperature-superconductivity-achieved
- https://www.rochester.edu/newscenter/rochester-sets-new-record-for-room-temperature-superconductor-455722/





Pingback: Google claims to create Time Crystals inside a Quantum Computer: What does it mean to the world & us? – The Inner Detail